Introduction
Have you ever noticed how hydrogen peroxide bubbles when applied to a cut or wound? This common observation sparks curiosity about the science behind the fizz. The bubbling reaction is not just a visual spectacle; it plays a significant role in both medical and educational contexts. At the heart of this reaction is an enzyme called catalase, which catalyzes the breakdown of hydrogen peroxide into water and oxygen. This process not only explains the bubbling effect but also highlights the body’s natural defense mechanisms against oxidative damage. As we delve deeper into the chemical reactions involved, we’ll uncover the fascinating interplay between hydrogen peroxide and catalase, setting the stage for a comprehensive understanding of why hydrogen peroxide bubbles.
The Chemical Composition of Hydrogen Peroxide
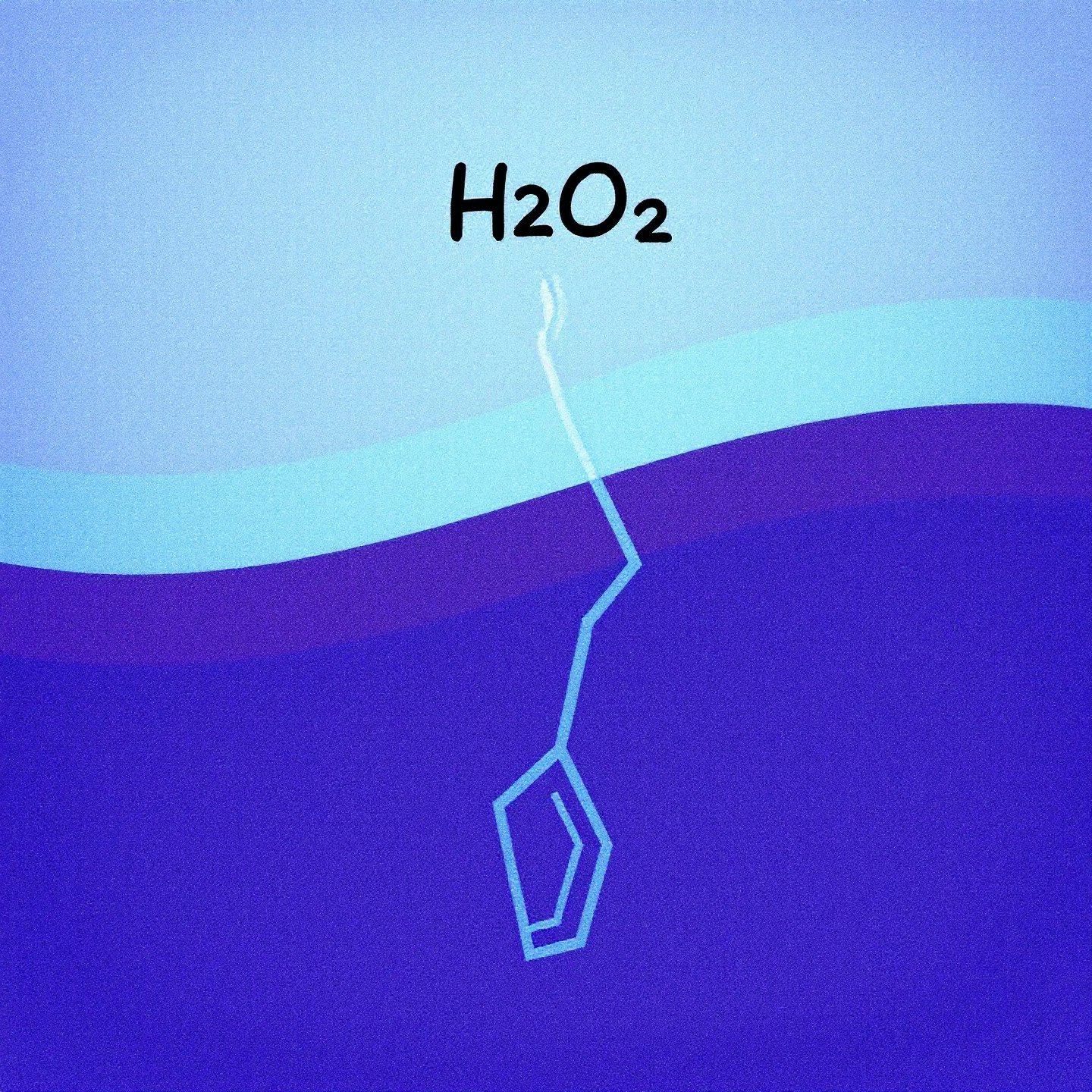
Hydrogen peroxide, with the chemical formula H₂O₂, is a simple yet fascinating molecule. At its core, it consists of two hydrogen atoms and two oxygen atoms, connected in a way that makes it highly reactive. The key to its reactivity lies in the oxygen-oxygen bond, known as a peroxide bond. This bond is notably weak and unstable, making hydrogen peroxide prone to decomposition into water (H₂O) and oxygen (O₂).
This decomposition process is not just a random occurrence; it’s a fundamental aspect of hydrogen peroxide’s chemical nature. When the peroxide bond breaks, it releases free radicals—highly reactive species that can interact with other substances. This characteristic makes hydrogen peroxide a powerful oxidizer, capable of breaking down organic materials and killing bacteria, which is why it’s commonly used as an antiseptic.
However, the decomposition of hydrogen peroxide doesn’t always require a catalyst. It can occur naturally over time, especially when exposed to light or heat. This is why hydrogen peroxide is typically stored in dark, opaque containers to slow down its decomposition. The presence of catalysts, such as the enzyme catalase found in living organisms, can significantly accelerate this process, leading to the rapid release of oxygen gas and the familiar bubbling effect.
Understanding the chemical composition of hydrogen peroxide provides a foundation for appreciating its behavior in various contexts, from medical applications to industrial processes. Its ability to decompose into water and oxygen not only explains the bubbling reaction but also underscores its utility as a cleaning agent, disinfectant, and even in educational experiments. As we explore further, we’ll see how this simple molecule plays a complex role in both science and everyday life.

Catalase: The Enzyme Behind the Bubbles
When you apply hydrogen peroxide to a wound, the immediate bubbling reaction is not just a random occurrence but a fascinating display of biochemistry in action. This reaction is primarily due to the presence of an enzyme called catalase, which plays a pivotal role in the decomposition of hydrogen peroxide (H₂O₂) into water (H₂O) and oxygen (O₂). Catalase is found in nearly all living organisms exposed to oxygen, including humans, where it is particularly abundant in the liver and blood cells.
Catalase functions as a catalyst, a substance that speeds up chemical reactions without being consumed in the process. In the case of hydrogen peroxide, catalase accelerates its breakdown into water and oxygen gas. This reaction is crucial because hydrogen peroxide is a byproduct of many metabolic processes and can be harmful to cells if it accumulates. By rapidly converting H₂O₂ into harmless substances, catalase protects cells from oxidative damage, which can lead to cell death and contribute to various diseases.
The efficiency of catalase is remarkable. A single molecule of this enzyme can convert millions of hydrogen peroxide molecules into water and oxygen every second. This high turnover rate is essential for maintaining cellular health, especially in tissues that are frequently exposed to oxidative stress. The oxygen gas released during this reaction is what causes the bubbling effect observed when hydrogen peroxide is applied to a wound. This visual cue not only indicates the presence of catalase but also serves as a reminder of the body’s intricate defense mechanisms against potential threats.
Beyond its biological importance, catalase’s role in the decomposition of hydrogen peroxide has practical applications in various fields, including medicine and industry. Understanding how catalase works not only sheds light on the science behind the bubbling reaction but also highlights the enzyme’s significance in protecting living organisms from oxidative damage. As we continue to explore the properties and functions of catalase, we gain deeper insights into the delicate balance that sustains life at the cellular level.
Hydrogen Peroxide as an Antiseptic: Pros and Cons
Hydrogen peroxide is widely recognized for its antiseptic properties, primarily due to its ability to release oxygen upon decomposition. This release of oxygen creates an environment that is inhospitable to anaerobic bacteria, effectively killing them and preventing infection. However, while hydrogen peroxide’s bubbling action is a sign of its antibacterial activity, it’s important to weigh its benefits against potential drawbacks, especially when used on wounds.
Pros of Using Hydrogen Peroxide as an Antiseptic
- Effective Against Bacteria: Hydrogen peroxide is effective at killing a wide range of bacteria, making it a popular choice for cleaning wounds and preventing infections.
- Readily Available: It is easily accessible and affordable, found in most households and pharmacies.
- Visual Feedback: The bubbling action provides immediate visual feedback that the solution is working, which can be reassuring to users.
Cons of Using Hydrogen Peroxide as an Antiseptic
- Damages Healthy Tissue: While it kills bacteria, hydrogen peroxide can also damage healthy cells, potentially delaying the healing process.
- Not Suitable for Deep Wounds: Its use is generally recommended for superficial wounds only, as it can cause tissue damage in deeper cuts.
- Potential for Overuse: Frequent use can lead to skin irritation and may disrupt the natural healing process.
| Aspect | Pros | Cons |
|---|---|---|
| Effectiveness | Kills a wide range of bacteria | Can damage healthy tissue |
| Availability | Easily accessible and affordable | Not suitable for deep wounds |
| User Feedback | Provides immediate visual feedback | Potential for overuse and skin irritation |
In conclusion, while hydrogen peroxide can be an effective antiseptic for minor wounds, its potential to harm healthy tissue and delay healing should not be overlooked. It’s essential to use it judiciously and consider alternative wound care methods, especially for more severe injuries. Understanding the pros and cons of hydrogen peroxide as an antiseptic allows for informed decisions about its use in wound care.
Observing the Bubbling Reaction in Action
When hydrogen peroxide is applied to a wound, the immediate fizzing and bubbling reaction is both fascinating and informative. This reaction is a direct result of the chemical breakdown of hydrogen peroxide (H₂O₂) into water (H₂O) and oxygen (O₂), a process catalyzed by the enzyme catalase. Catalase is abundantly present in the blood and damaged cells of living organisms, making it readily available to interact with hydrogen peroxide upon contact.
The process begins when hydrogen peroxide comes into contact with the catalase enzyme. Catalase accelerates the decomposition of H₂O₂ by breaking the weak oxygen-oxygen bond, leading to the formation of water and oxygen gas. The release of oxygen gas is what causes the visible bubbling effect. This reaction is not only rapid but also highly efficient, with a single catalase molecule capable of decomposing millions of hydrogen peroxide molecules per second.
From a visual perspective, the bubbling reaction serves as an indicator of catalase activity. The more vigorous the bubbling, the higher the concentration of catalase present in the tissue. This is why hydrogen peroxide bubbles more noticeably on fresh wounds or areas with significant tissue damage, where catalase is released from ruptured cells. In contrast, applying hydrogen peroxide to unbroken skin typically results in little to no bubbling, as the catalase remains contained within intact cells.
Beyond its visual appeal, the bubbling reaction has practical implications. The oxygen gas released during the decomposition of hydrogen peroxide helps to cleanse the wound by lifting away debris and dead tissue. Additionally, the oxygen-rich environment created by the reaction can inhibit the growth of anaerobic bacteria, further aiding in wound disinfection. However, it’s important to note that while the bubbling action is a sign of hydrogen peroxide’s antibacterial properties, excessive use can damage healthy tissue and delay healing.
Understanding the science behind the bubbling reaction not only satisfies curiosity but also underscores the importance of using hydrogen peroxide judiciously. The interplay between hydrogen peroxide and catalase highlights the body’s natural defense mechanisms and provides a glimpse into the intricate biochemical processes that occur at the cellular level. As we observe the fizzing and bubbling, we are reminded of the delicate balance between effective wound care and the preservation of healthy tissue.

Beyond Wound Care: Other Uses of Hydrogen Peroxide
While hydrogen peroxide is commonly known for its antiseptic properties, its bubbling reaction has found a place in educational and experimental settings. One of the most popular demonstrations of hydrogen peroxide’s decomposition is the ‘Elephant’s Toothpaste’ experiment. This visually striking experiment showcases the rapid breakdown of hydrogen peroxide into water and oxygen, producing a large volume of foam that resembles toothpaste being squeezed from a tube—hence the name.
The ‘Elephant’s Toothpaste’ experiment typically involves mixing hydrogen peroxide with a catalyst, such as potassium iodide or yeast, and a bit of dish soap. The catalyst accelerates the decomposition of hydrogen peroxide, releasing oxygen gas. The dish soap traps the oxygen bubbles, creating a thick foam that expands rapidly. This reaction not only provides a dramatic visual display but also serves as an excellent teaching tool for understanding chemical reactions, catalysts, and the properties of gases.
Beyond the classroom, hydrogen peroxide’s bubbling property is utilized in various other applications. For instance, it is used in the cleaning industry to remove stains and disinfect surfaces. The oxygen released during its decomposition helps to break down organic materials, making it effective for cleaning purposes. Additionally, hydrogen peroxide is used in hair bleaching and teeth whitening products, where its oxidizing properties help to lighten colors.
In the realm of gardening, hydrogen peroxide can be used to oxygenate soil and promote plant health. When diluted and applied to soil, it releases oxygen, which can help to aerate the soil and encourage root growth. This application leverages the same bubbling reaction observed in wound care and educational experiments, demonstrating the versatility of hydrogen peroxide’s chemical properties.
Understanding the diverse applications of hydrogen peroxide’s bubbling reaction not only highlights its utility beyond wound care but also underscores the importance of chemical reactions in everyday life. Whether it’s creating a foamy spectacle in a science experiment or aiding in the growth of plants, hydrogen peroxide’s ability to decompose into water and oxygen continues to find new and innovative uses.
Safety Considerations When Using Hydrogen Peroxide
While hydrogen peroxide is a common household item known for its antiseptic properties, it’s crucial to understand the safety concerns associated with its use. The bubbling reaction that makes hydrogen peroxide effective against bacteria can also pose risks to healthy tissue, especially when used improperly or in high concentrations.
Potential Risks of Hydrogen Peroxide
- Tissue Irritation: Hydrogen peroxide can cause irritation to the skin and mucous membranes, particularly at concentrations higher than 3%. This irritation can manifest as redness, stinging, or even blistering if left on the skin for extended periods.
- Delayed Wound Healing: While hydrogen peroxide effectively kills bacteria, it can also damage the cells necessary for wound healing. This can lead to delayed recovery and increased risk of scarring.
- Respiratory and Ocular Risks: Inhalation of hydrogen peroxide vapors can irritate the respiratory tract, while contact with the eyes can cause corneal burns. It’s essential to use hydrogen peroxide in well-ventilated areas and to wear protective eyewear when handling concentrated solutions.
Guidelines for Safe Use
To minimize risks, it’s important to follow these guidelines when using hydrogen peroxide:
- Use Appropriate Concentrations: For household use, a 3% concentration is generally safe. Higher concentrations should be handled with care and used only in specific applications, such as industrial cleaning or hair bleaching, under professional guidance.
- Avoid Overuse: Limit the use of hydrogen peroxide on wounds to prevent tissue damage. For minor cuts and scrapes, cleaning with soap and water is often sufficient.
- Consult Healthcare Professionals: For more serious injuries or if you’re unsure about how to treat a wound, seek advice from a healthcare professional. They can recommend safer alternatives and provide appropriate treatment.
When to Seek Medical Attention
If you experience severe irritation, prolonged discomfort, or any signs of an allergic reaction after using hydrogen peroxide, it’s important to seek medical attention immediately. Symptoms such as difficulty breathing, chest pain, or significant skin damage warrant prompt medical evaluation.
By understanding the safety considerations and adhering to proper usage guidelines, you can harness the benefits of hydrogen peroxide while minimizing potential risks. Always prioritize safety and consult healthcare professionals when in doubt to ensure effective and safe wound care.
Conclusion
Throughout this exploration of hydrogen peroxide’s bubbling reaction, we’ve uncovered the fascinating interplay between chemistry and biology that makes this everyday phenomenon possible. From the initial observation of hydrogen peroxide fizzing on a wound to the deeper understanding of catalase’s role in catalyzing the breakdown of H₂O₂ into water and oxygen, we’ve seen how this simple reaction serves as a window into the complex world of biochemical processes.
The chemical composition of hydrogen peroxide, with its reactive oxygen-oxygen bond, sets the stage for its decomposition, a process that is both rapid and efficient in the presence of catalase. This enzyme, found abundantly in living cells, not only accelerates the reaction but also protects cells from oxidative damage, highlighting the delicate balance that sustains life at the cellular level.
We’ve also examined the practical applications of hydrogen peroxide’s bubbling property, from its use as an antiseptic to its role in educational experiments like the ‘Elephant’s Toothpaste’ demonstration. These applications underscore the versatility of hydrogen peroxide and its importance in both medical and scientific contexts.
However, it’s crucial to approach the use of hydrogen peroxide with caution, understanding both its benefits and potential risks. While it can be an effective tool for cleaning and disinfecting, its ability to damage healthy tissue and delay wound healing necessitates careful and informed use.
In conclusion, the bubbling reaction of hydrogen peroxide is more than just a visual spectacle; it’s a testament to the intricate and interconnected nature of chemical and biological processes. By appreciating the science behind this everyday phenomenon, we can use hydrogen peroxide safely and effectively, harnessing its properties to our advantage while respecting its potential to cause harm. Let this exploration inspire a deeper curiosity about the world around us and a greater appreciation for the science that shapes our daily lives.
Frequently Asked Questions
1. Is hydrogen peroxide still good if it bubbles?
Yes, if hydrogen peroxide bubbles when poured into a sink, it indicates that it’s still active. However, its effectiveness decreases over time, especially once the bottle is opened, so it’s best used within 1–6 months.
2. Why does hydrogen peroxide bubble on cuts and infections but not on healthy skin?
Hydrogen peroxide bubbles on cuts and infections due to the presence of catalase, an enzyme found in blood and damaged cells that accelerates its breakdown into water and oxygen. Healthy skin lacks exposed catalase, resulting in minimal to no bubbling.
3. Do hydrogen peroxide bubbles mean infection?
While bubbling indicates the presence of catalase, which is abundant in blood and damaged cells, it doesn’t necessarily mean an infection is present. It’s a sign of the chemical reaction between hydrogen peroxide and catalase.
4. Why does hydrogen peroxide fizz and make white foam when poured on a cut?
The fizzing and white foam occur as hydrogen peroxide decomposes into water and oxygen gas in the presence of catalase. The oxygen gas forms bubbles, and the foam is created when these bubbles are trapped by the liquid, providing a visual indication of the reaction.
5. What is the role of catalase in hydrogen peroxide decomposition?
Catalase is an enzyme that significantly accelerates the breakdown of hydrogen peroxide into water and oxygen. This reaction is crucial for protecting cells from oxidative damage and is responsible for the bubbling effect observed when hydrogen peroxide is applied to wounds.