Introduction
When you think of household cleaning agents, hydrogen peroxide likely comes to mind. Known for its disinfecting properties, this versatile chemical also boasts bleaching capabilities. But does hydrogen peroxide bleach clothes effectively and safely? This question is crucial for anyone looking to brighten and whiten their fabrics without resorting to harsh chemicals.
Hydrogen peroxide, a common item in many medicine cabinets, is celebrated for its ability to kill bacteria and viruses. However, its role in laundry is equally noteworthy. As an oxidizing agent, hydrogen peroxide can break down stains and brighten fabrics, making it a popular choice for those seeking a gentler alternative to traditional bleach.
But how does it work on different types of fabrics? And what precautions should you take to ensure it doesn’t damage your clothes? This article delves into the science behind hydrogen peroxide’s bleaching effects, offering practical advice for incorporating it into your laundry routine. Whether you’re dealing with stubborn stains or simply aiming to refresh your wardrobe, understanding the role of hydrogen peroxide in laundry can help you achieve cleaner, brighter clothes safely and effectively.
How Hydrogen Peroxide Works as a Bleaching Agent
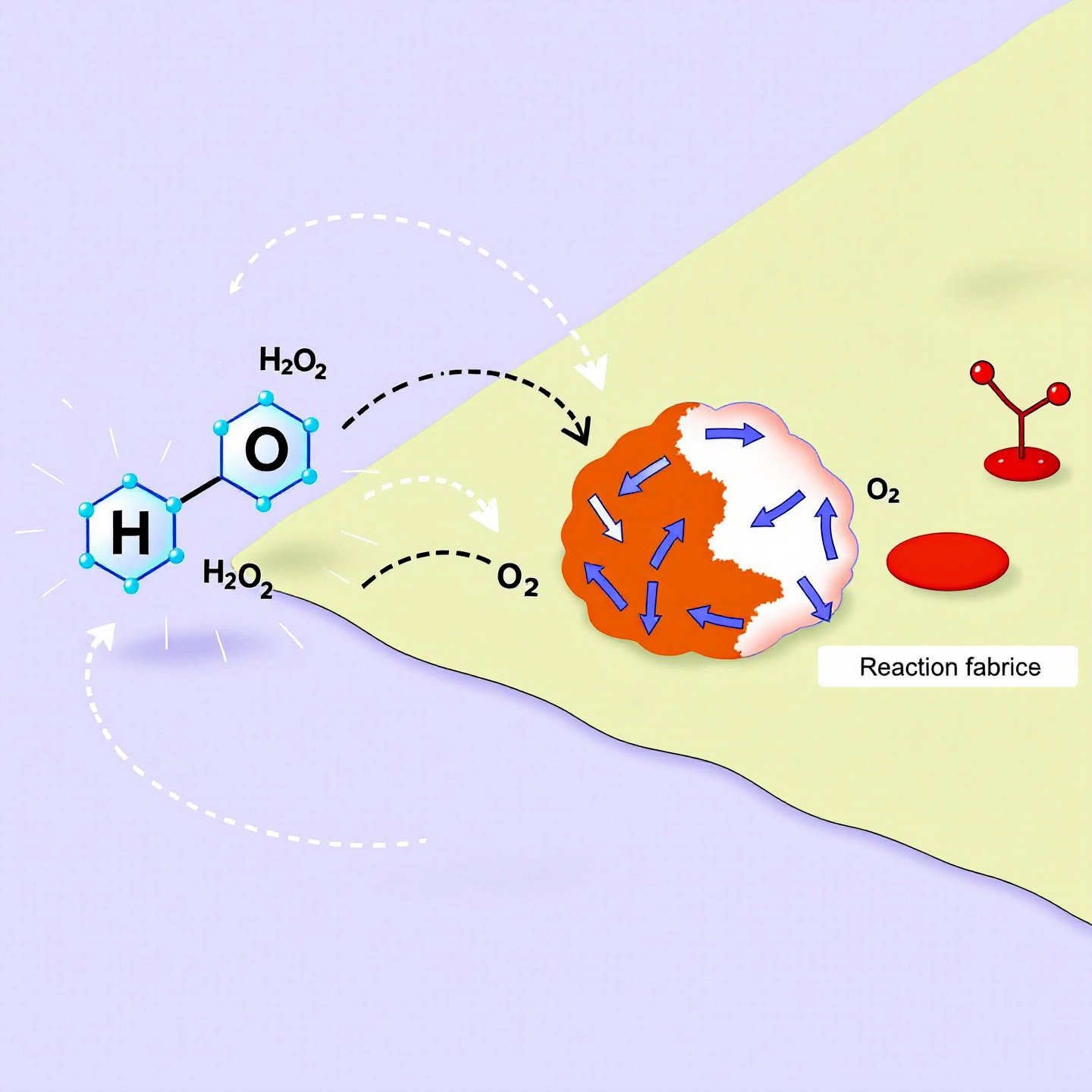
Hydrogen peroxide’s ability to bleach clothes lies in its chemical structure and behavior. When hydrogen peroxide (H2O2) comes into contact with fabrics, it decomposes into water (H2O) and oxygen (O2). This decomposition releases reactive oxygen species, highly reactive molecules that play a pivotal role in the bleaching process.
These reactive oxygen species target chromophores, the parts of molecules responsible for color. By breaking the chemical bonds within chromophores, hydrogen peroxide effectively removes or lightens stains and discoloration. This mechanism is particularly effective on organic stains, such as those from food, blood, or grass, which contain chromophores that are susceptible to oxidation.
However, the effectiveness of hydrogen peroxide as a bleaching agent can vary depending on the fabric type and the concentration of the solution used. For instance, cotton and linen, which are more porous, tend to respond well to hydrogen peroxide bleaching. On the other hand, synthetic fabrics like polyester and nylon may require higher concentrations or longer exposure times to achieve noticeable results.
It’s also important to note that while hydrogen peroxide is a powerful bleaching agent, it is generally milder than chlorine bleach. This makes it a safer option for colored fabrics, as it is less likely to cause fading or damage. However, as with any bleaching agent, it’s crucial to perform a spot test on a small, inconspicuous area of the fabric before applying it more broadly.
Understanding the science behind how hydrogen peroxide bleaches clothes can help you use it more effectively in your laundry routine. By knowing which fabrics and stains it works best on, you can achieve brighter, cleaner clothes without the risk of damage.
Suitable Fabrics for Hydrogen Peroxide Bleaching
When considering hydrogen peroxide for laundry, it’s essential to understand how different fabrics react to this bleaching agent. Not all materials are created equal, and their response to hydrogen peroxide can vary significantly. Here’s a breakdown of how hydrogen peroxide interacts with various fabric types:
Cotton and Linen
Cotton and linen are natural fibers that generally respond well to hydrogen peroxide. These fabrics are more porous, allowing the hydrogen peroxide to penetrate and break down stains effectively. Whether you’re dealing with stubborn stains or looking to brighten whites, hydrogen peroxide can be a safe and effective choice for cotton and linen garments.
Polyester and Nylon
Synthetic fabrics like polyester and nylon can also be treated with hydrogen peroxide, but they may require a bit more attention. These materials are less porous than natural fibers, so achieving noticeable results might take higher concentrations or longer exposure times. Always perform a spot test to ensure the fabric’s colorfastness before proceeding with a full application.
Delicate Fabrics: Silk and Wool
Delicate fabrics such as silk and wool require extra caution. Hydrogen peroxide can be too harsh for these materials, potentially causing damage or discoloration. If you must use hydrogen peroxide on silk or wool, dilute it significantly and apply it sparingly. However, it’s generally advisable to avoid using hydrogen peroxide on these fabrics altogether to preserve their integrity and appearance.
Colorfastness Testing
Before using hydrogen peroxide on any fabric, it’s crucial to perform a colorfastness test. Apply a small amount of hydrogen peroxide to an inconspicuous area and wait for a few minutes. If there’s no color change or damage, it’s likely safe to proceed. This simple step can save you from potential fabric disasters.
Understanding how hydrogen peroxide affects different fabrics can help you make informed decisions in your laundry routine. By knowing which materials are safe to bleach and which ones to avoid, you can achieve brighter, cleaner clothes without risking damage.

Recommended Concentrations and Application Techniques
When using hydrogen peroxide to bleach clothes, the concentration of the solution and the method of application are key factors that determine both effectiveness and safety. Typically, a 3% hydrogen peroxide solution, commonly found in drug stores, is recommended for laundry purposes. This concentration is strong enough to break down stains and brighten fabrics without causing significant damage.
Step-by-Step Application Guide
1. Spot Testing: Before applying hydrogen peroxide to any garment, it’s crucial to perform a spot test. Apply a small amount of the solution to an inconspicuous area of the fabric and wait for about 10 minutes. If there’s no discoloration or damage, it’s safe to proceed.
2. Dilution: For general laundry use, dilute the hydrogen peroxide with water. A common ratio is one part hydrogen peroxide to two parts water. This dilution helps to minimize the risk of fabric damage while still providing effective bleaching.
3. Application: For spot treatments, apply the diluted hydrogen peroxide directly to the stain using a clean cloth or sponge. Allow it to sit for 10-15 minutes before rinsing thoroughly with cold water. For overall whitening, add the diluted solution to the washing machine’s bleach dispenser or directly into the wash cycle.
4. Rinsing: After the application, rinse the treated area or the entire garment thoroughly with cold water to remove any residual hydrogen peroxide. This step is essential to prevent any potential fabric weakening or discoloration over time.
Recommended Concentrations for Different Needs
For light stains and general brightening, a 3% solution is usually sufficient. However, for tougher stains or more significant whitening needs, you might consider a slightly higher concentration, but always ensure it’s safe for the fabric type. Remember, higher concentrations can increase the risk of fabric damage, so use them sparingly and with caution.
By following these recommended concentrations and application techniques, you can safely and effectively use hydrogen peroxide to bleach your clothes, achieving brighter and cleaner results without compromising fabric integrity.
Potential Risks and Precautions
While hydrogen peroxide is a powerful ally in the fight against stains and dullness, it’s not without its risks. Understanding these potential pitfalls can help you use this versatile chemical safely and effectively in your laundry routine.
Colorfastness: A Crucial First Step
Before you even think about applying hydrogen peroxide to your clothes, it’s essential to test for colorfastness. This simple step can save you from the heartbreak of ruined garments. Apply a small amount of hydrogen peroxide to an inconspicuous area of the fabric and wait for about 10 minutes. If there’s no color change or damage, it’s likely safe to proceed. This precaution is especially important for colored fabrics, as hydrogen peroxide can cause fading or discoloration.
Fabric Weakening: A Hidden Danger
Another risk to consider is the potential for fabric weakening. Hydrogen peroxide, especially in higher concentrations, can break down the fibers in your clothes over time, leading to thinning and eventual tearing. To minimize this risk, always dilute hydrogen peroxide with water before use and avoid using it on delicate fabrics like silk and wool. Additionally, ensure thorough rinsing after application to remove any residual hydrogen peroxide that could continue to break down fibers.
Skin Protection: Don’t Forget Your Hands
While hydrogen peroxide is generally safe for fabrics, it can be harsh on your skin. Prolonged exposure can lead to irritation or even chemical burns. Always wear gloves when handling hydrogen peroxide, especially if you’re using it in concentrated forms or for extended periods. This simple precaution can protect your skin from unnecessary harm.
Minimizing Risks: Practical Tips
- Dilute Properly: Always dilute hydrogen peroxide with water to reduce its strength and minimize potential damage to fabrics and skin.
- Spot Test: Never skip the colorfastness test. It’s a small step that can prevent significant problems.
- Use Sparingly: Avoid overuse. Hydrogen peroxide is effective in small amounts, and excessive use can lead to fabric weakening and color fading.
- Rinse Thoroughly: After application, rinse the treated area or garment thoroughly to remove any residual hydrogen peroxide.
By understanding and addressing these potential risks, you can safely incorporate hydrogen peroxide into your laundry routine. With the right precautions, this versatile chemical can help you achieve brighter, cleaner clothes without the worry of damage or harm.

Alternative Bleaching Agents and Environmental Considerations
While hydrogen peroxide is a popular choice for bleaching clothes, it’s not the only option available. For those seeking alternatives, there are several effective and environmentally friendly options to consider. Each of these alternatives offers unique benefits and can be used depending on your specific laundry needs and fabric types.
Oxygen-Based Bleach
Oxygen-based bleach, such as OxiClean, is a gentler alternative to chlorine bleach and hydrogen peroxide. It works by releasing oxygen ions that break down stains and brighten fabrics. Unlike chlorine bleach, oxygen-based bleach is safe for colored fabrics and does not produce harmful fumes. It’s particularly effective for removing organic stains and can be used in both standard and high-efficiency washing machines.
Lemon Juice
Lemon juice is a natural bleaching agent that can be used to brighten whites and remove stains. Its acidic nature helps break down stains and can be particularly effective on cotton and linen. To use lemon juice, simply add one cup to your washing machine during the wash cycle. For tougher stains, you can soak the affected area in a mixture of lemon juice and water before washing.
Baking Soda
Baking soda is another eco-friendly option that can enhance the cleaning power of your laundry detergent. Adding half a cup of baking soda to your wash can help whiten clothes and remove odors. Baking soda is safe for all fabric types and can be used in conjunction with other bleaching agents for added effectiveness.
Environmental Benefits of Hydrogen Peroxide
When compared to chlorine-based bleaches, hydrogen peroxide offers significant environmental advantages. Unlike chlorine bleach, which can produce harmful by-products like dioxins, hydrogen peroxide breaks down into water and oxygen, making it a more sustainable choice. This biodegradability ensures that hydrogen peroxide does not contribute to long-term environmental pollution, making it a safer option for both your clothes and the planet.
By exploring these alternative bleaching agents and understanding the environmental benefits of hydrogen peroxide, you can make more informed choices for your laundry routine. Whether you opt for oxygen-based bleach, lemon juice, baking soda, or stick with hydrogen peroxide, each option offers a way to achieve brighter, cleaner clothes while minimizing environmental impact.
Conclusion
Hydrogen peroxide stands out as a versatile and effective bleaching agent for clothes, offering a safer alternative to traditional chlorine bleach. Throughout this article, we’ve explored its science-backed bleaching mechanism, suitability for various fabrics, and practical application techniques. By understanding the importance of fabric types, proper concentrations, and safe usage, you can confidently incorporate hydrogen peroxide into your laundry routine to achieve brighter, cleaner clothes without risking damage.
Key takeaways include:
- Fabric Compatibility: Hydrogen peroxide works best on natural fibers like cotton and linen, while synthetic and delicate fabrics require extra caution.
- Safe Application: Always perform a colorfastness test and dilute hydrogen peroxide to minimize risks of fabric weakening or discoloration.
- Environmental Benefits: Unlike chlorine bleach, hydrogen peroxide breaks down into water and oxygen, making it an eco-friendly choice for sustainable laundry practices.
As you embrace hydrogen peroxide for your laundry needs, consider pairing it with other eco-conscious habits, such as exploring renewable energy solutions like solar panels. Renewable Energy Nexus offers a wide range of solar panel products designed to reduce your carbon footprint and energy costs. By integrating sustainable practices into your daily life, you contribute to a cleaner, brighter future for all.
For more information on solar panels and renewable energy solutions, visit Renewable Energy Nexus.
Frequently Asked Questions
1. Will hydrogen peroxide strip color from clothes?
Hydrogen peroxide can lighten colors on fabrics, especially if used in high concentrations or on non-colorfast materials. Always perform a spot test before full application.
2. Can hydrogen peroxide be used on all types of fabric?
Hydrogen peroxide is safe for many fabrics like cotton and linen but should be used cautiously on synthetics and avoided on delicate materials such as silk and wool.
3. How does hydrogen peroxide compare to chlorine bleach?
Hydrogen peroxide is a milder bleaching agent than chlorine bleach, making it safer for colored fabrics and environmentally friendly as it breaks down into water and oxygen.
4. What is the best way to apply hydrogen peroxide to clothes?
For best results, dilute hydrogen peroxide with water, apply it directly to stains, let it sit for 10-15 minutes, then rinse thoroughly. Always test on a small area first.
5. Are there eco-friendly alternatives to hydrogen peroxide for bleaching clothes?
Yes, alternatives include oxygen-based bleaches, lemon juice, and baking soda, which offer effective bleaching with minimal environmental impact.



